Vapotherm, a leader in respiratory care solutions, partnered with Re:Build Fikst to develop its next-generation High-Velocity Nasal Insufflation (HVNI) product line. HVNI is used in the management of respiratory distress from various causes, including COPD, pneumonia, and other respiratory infections. Together, Vapotherm and Re:Build Fikst engineered the HVT 2.0, bringing significant improvements in ease of use and consistency of performance.
The device works with a disposable patient circuit (DPC), also co-developed with Re:Build Fikst. The DPC uses induction power transfer for water vapor generation and capacitive water-level sensing, both of which are part of the suite of solutions employed to achieve full isolation of the capital device and patient circuit.
The key challenge arose from Vapotherm’s need to develop a product which delivers their therapeutic technology to care areas that lack hospital air. With its integrated internal blower, hot-swappable battery system, and wireless integration into hospital information systems, the HVT 2.0 significantly expands the areas within a hospital where the device can be used.
The project combined development across electrical, software, and industrial design disciplines, and demanded a process that combined iterative design, rapid prototyping, and rigorous testing to international safety and performance standards. Additionally, establishing a scalable production process that included comprehensive documentation and training for manufacturing staff was paramount. These multifaceted challenges necessitated a collaborative approach between Vapotherm and Re:Build Fikst’s engineering, manufacturing, and quality assurance teams.
Image © Vapotherm
Image © Vapotherm
The HVT 2.0 development required a close collaboration between Vapotherm and Re:Build Fikst. The HVT 2.0 was developed under an FDA compliant product development process and adheres to numerous international product performance and safety standards. Within these constraints, Vapotherm and Re:Build Fikst were able to advance rapidly through the stages of concept development, design, and validation. Employing a carefully conceived development strategy with clear and measurable objectives for each stage of development, rapid prototyping techniques, and frequent feedback from user-centered studies, the result has been enthusiastically received by clinicians and patients alike.
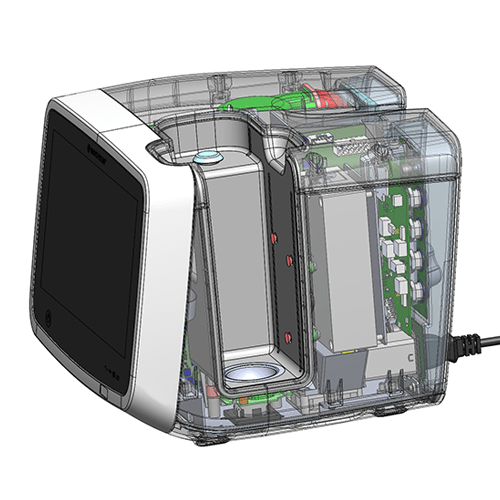

Image © Vapotherm
The collaborative efforts between Vapotherm and Re:Build Fikst resulted in a state-of-the-art device that not only met but exceeded performance benchmarks. The HVT 2.0 demonstrated enhanced reliability, contributing to better patient outcomes by effectively reducing the need for endotracheal intubation. The streamlined production process, supported by rigorous documentation and targeted staff training, has laid the groundwork for scalable manufacturing and consistent production quality. This meticulous approach has strengthened Vapotherm’s market position and reinforced their reputation for technical excellence in respiratory care. Moreover, the data-backed reliability testing across all critical components provided quantifiable assurance to both clinicians and healthcare administrators, cementing the device’s status as a breakthrough in non-invasive respiratory therapy. Through this project, Vapotherm not only advanced its product line but also set a new standard in the careful balance between high performance and manufacturing efficiency.
Were you happy with our work? We would love to feature a testimonial quote about your experience, if you’re willing.
— Author
Image © Vapotherm
Fill out the form below and someone from the Re:Build Fikst team will be in touch with you shortly.